| |
Isolation of spectral variants of GFP and other fluorescent proteins has made it possible to perform multi-labeling experiments, test for molecular proximity using Forstner Resonance Energy Transfor (FRET), and to explore molecular dynamics with photoactivatible and photo-convertible probes. Some of the more popular fluorescent proteins and their applications are described below: |
|
| |
|
 Chromophores Chromophores |
| |
| |
- EGFP (GFPmut1). The original enhanced green fluorescent protein and still one of the most useful probes. The chromophore mutation, GFPmut1 was isolated by Cormack et al using FACS sorting. It is characterized by red-shifted excitation and emission peaks in comparison to wildtype GFP, a greatly enhanced extinction coefficient, a significantly faster chromophore formation time, and is spectrally similar to the S65T chromophore (it also contains the S65T mutation). The GFPmut1 chromophore is available from Clontech in a codon-optimized version (EGFP) that eliminates the Arabidopsis cryptic splice site discovered by Haseloff and colleagues.
- Venus and Citrine YFP's. Isloated by Atushi Miyawaki's lab and Roger Tsien's lab, respectively, these two proteins are further red-shifted variants of GFP. They both have enhanced brightness compared with EGFP and have lower pKa's, making them less sensitive to changes in pH under many physiological condistions. However, these chromophores are also somewhat less photostable than EGFP, limiting their application in experiments requiring sustained or repeated excitation.
- ECFP (W7) and Cerulean. The "cyan" chromophore was also isolated by Roger Tsien's group and was shown to form an effective pair with YFP for FRET experiments. Older confocal systems often do not have an appropriate laser line for chromophore excitation (442 HeCd, 440-449 Diode or the 457 line of more powerful argon ion laser), although most newer systems do. A codon-optimized version is available from Clontech as ECFP. Cerulean is an improved version of ECFP developed by the Piston lab. it has improved qunatum yield and a higher extinction coefficient to yield a chromophore with approximately 2.5x the brightness of ECFP. Further, it is more photostable and has a decay curve better fit by a single exponent, making it the cyan chromophore of choice for FRET applications, especially when lifetime imaging (FLIM) is used.
- mCherry and tdTomato. The red fluorescing ancestor for these proteins was isolated from Discosoma coral by the Tsien lab. A series of improvements resulted in variants that do not oligomerize, have improved brightness and which fluoresce over a wide range of the visible spectrum. mCherry is often considered the chromophore of choice when a single fluoecent protein tag is desired, whereas tdTomato is thus far the brighttest fluorecent protein but at the cost of beng a larger tag consisting of a tandem protein. In plant systems, their emission spectra overlaps with that of plastid autofluorescence, but this is not a significant issue for many kinds of experiments.
|
|
| |
 |
|
| |
|
|
| |
 |
|
| |
|
|
| |
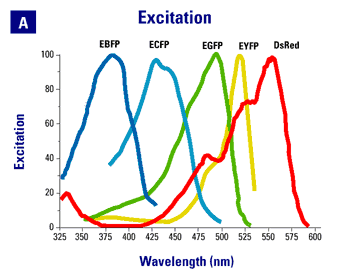
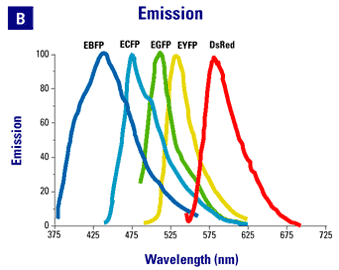
Spectra are from the Clontech web site. |
|
| |
|
|
 Strategies Strategies |
|
| |
|
|
| |
- EGFP and Citrine/Venus. GFP and YFP can be at least partially discriminated on the basis of careful filter selection. However, both excitation and emission spectra are fairly close together, limiting the discrimination possible and requiring difficult compromises to be made between brightness and discrimination. Spectral scanning and unmixing, such as is possible on scanning confocal units form Zeiss, Leica and Nikon, can improve separation of these chromophores.
- Citrine/Venus and Cerulean. The excitation and emission spectra for CFP+YFP are much better separated than is the case for GFP+YFP. However, many older point scanning confocal systems do not have an appropriate laser line for CFP excitation. The Lippencot-Schwartz lab has overcome this problem by using a 415-line form a Kr laser to differentially excite CFP over YFP (Presley et al.). We have installed a 442 nm HeCd laser on our older BioRad 1024 point scanning confocal for CFP excitation. On our newer Leica SP5 and on our spinning disk systems we use 443 diode lasers. The 443 units ship with a wide range of actual outputs. We have had to swap out two such units, which originally output at 449 nm, for units that actually output at 443 nm in order to use commonly available commercial filter sets. While much better than GFP+YFP, the overlap in emmission spectra between CFP and YFP still requires that we acquire images sequentially rather than simultaneously for good chromophore separation. For very dynamic events, such as colocalization studies in rapidly stremaing small organelles, sequential imaging can be problematic.
- EGFP and mCherry/tdTomato. A robust combination for two-color imaging studies. The separation of of both excitation and emmission spectra allow for simultaneous imaging of the two chromophores.
|
|
| |
|
|
| |
|
|
| |
 Chromophores
Chromophores

 Strategies
Strategies
